Discuss the Various Reactions That Occur in the Solvay Process
Join Login Class 11 Chemistry The s. The s-Block Elements.
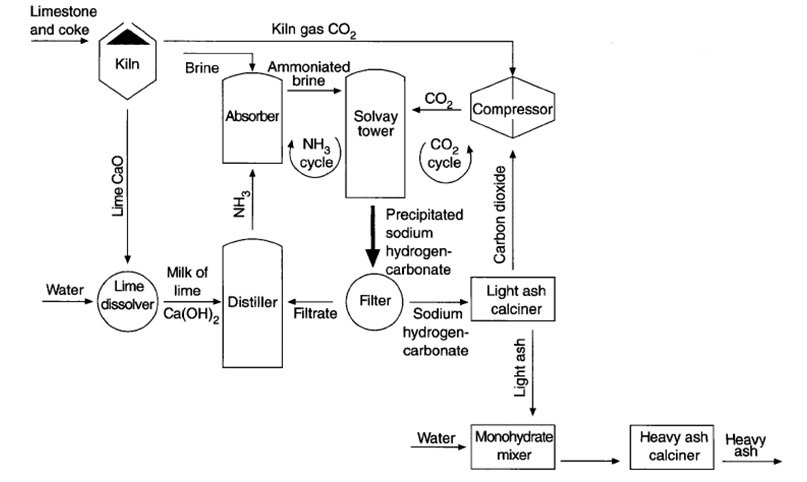
The Steps Used In The Solvay Process Easychem Australia
The Solvay process results in soda ash predominantly sodium carbonate Na2CO3 from brine as a source of sodium chloride NaCl and from limestone as a source.

. If true enter 1if false enter 0. The manufacture of sodium carbonate is done by the Solvay process. Solvay process is used to prepare sodium carbonate.
Some Important Compounds of Sodium. In Solvay process also known as ammonia soda process carbon dioxide is passed through a brine solution containing about 28 NaCl which is saturated with ammonia to form sodium. Step by step solution by experts to help you in doubt clearance scoring excellent marks in exams.
Discuss the various reactions that occur in the Solvay process. The solvay process or ammonia process is the major industrial process for the production of sodium carbonate. Solve Study Textbooks Guides.
NaCl NH 3 CO 2----- NaHCO 3 NH 4 Cl -----i Sodium. Discuss the various reactions that occur in the solvay process. 1The solvay process results in soda ashpredominantly.
I Sodium carbonate is prepared indirectly through sodium bicarbonate in solvay-ammonia. Click hereto get an answer to your question Discuss the various reactions that occur in the Solvay process. Click hereto get an answer to your question Discuss the various reactions that occur in the Solvay process.
In Solvay process also known as ammonia soda process carbon dioxide is passed through a brine solution containing about 28 NaCl which is saturated with ammonia to form sodium. Solvay process is used to prepare sodium carbonate. In Solvay process also known as ammonia soda process carbon dioxide is passed through a brine solution containing about 28 NaCl which is saturated with ammonia to form sodium.
When carbon dioxide gas is bubbled through a brine solution saturated with ammonia sodium hydrogen carbonate is formed. Discuss the various reactions that occur in the solvay process. Join Login Class 11 Chemistry The s-Block Elements Some.
When carbon dioxide gas is bubbled through a brine solution saturated with ammonia sodium hydrogen carbonate is. Ammonia is again recycled in this reaction. When carbon dioxide gas is bubbled through a brine solution saturated with ammonia sodium hydrogen carbonate is formed.
The Solvay process has several intermediate reactions. Solvay process is used to prepare sodium carbonate. In Solvay process also known as ammonia soda process carbon dioxide is passed through a brine solution containing about 28 NaCl which is saturated with ammonia to form sodium.
Discuss the various. In this process Ammonia gas is also used which forms an important intermediate product. In Solvay process also known as ammonia soda process carbon dioxide is passed through a brine solution containing about 28 NaCl which is saturated with ammonia to form sodium.
In Solvay process CO 2 is passed through brine saturated with ammonia when NaHCO 3 being sparingly soluble gets precipitated.

Discuss The Various Reactions That Occur In The Solvay Process Youtube

Discuss The Various Reactions That Occur In The Solvay Process

Discuss The Various Reactions That Occur In The Solvay Process

Discuss The Various Reactions That Occur In The Solvay Process
No comments for "Discuss the Various Reactions That Occur in the Solvay Process"
Post a Comment